Clinical data on the Heliosphere balloon and its competitors
Efficacy
A quick review of recent intragastric balloon efficiency data shows that the Heliosphere1,2,3,4,5 balloon offers significative efficacy for the treatment of obesity. The consensual threshold of 25% of Excess Weight Loss is clearly reached by patients treated by the Heliosphere balloon.
| Study | Year | Number of patients with Heliosphere | Mean Implantation duration | Mean BMI at insertion (kg/m2) | Mean BMI loss (kg/m2) | Mean Excess Weight Loss (%) | Mean Weight Loss (kg) |
|---|---|---|---|---|---|---|---|
| Erdem, H. | 2016 | 75 | 26 weeks | 41,6 ± 6,7 | 5,7 | 33.2% | Not reported |
| Palmisano, S. | 2016 | 58 | 24 weeks | 39,6 ± 6,9 | 3,6 ± 2,3 | 28.1% ± 20 | 10,1 ± 6,5 |
| Coenye | 2017 | 250 | 24 Weeks | NR | 15(0-42kg) | NR | NR |
| Romney, R. | 2020 | 110 | 29 weeks | 35,2 ± 4,3 | 4,4 ± 2,1 | 48% ± 33 | 12,2 ± 5,7 |
| Study | Erdem, H. |
|---|---|
| Year | 2016 |
| Number of patients with Heliosphere | 75 |
| Mean Implantation duration | 26 weeks |
| Mean BMI at insertion (kg/m2) | 41,6 ± 6,7 |
| Mean BMI Loss (kg/m2) | 5,7 |
| Mean Excess Weight Loss (%) | 33.2% |
| Mean Weight Loss (kg) | Not reported |
| Study | Palmisano, S. |
|---|---|
| Year | 2016 |
| Number of patients with Heliosphere | 58 |
| Mean Implantation duration | 24 weeks |
| Mean BMI at insertion (kg/m2) | 39,6 ± 6,9 |
| Mean BMI Loss (kg/m2) | 3,6 ± 2,3 |
| Mean Excess Weight Loss (%) | 28.1% ± 20 |
| Mean Weight Loss (kg) | 10,1 ± 6,5 |
| Study | Coenye |
|---|---|
| Year | 2017 |
| Number of patients with Heliosphere | 250 |
| Mean Implantation duration | 24 Weeks |
| Mean BMI at insertion (kg/m2) | NR |
| Mean BMI Loss (kg/m2) | 15(0-42kg) |
| Mean Excess Weight Loss (%) | NR |
| Mean Weight Loss (kg) | NR |
| Study | Romney, R. |
|---|---|
| Year | 2020 |
| Number of patients with heliosphere | 110 |
| Mean Implantation duration | 29 weeks |
| Mean BMI at insertion (kg/m2) | 35,2 ± 4,3 |
| Mean BMI loss (kg/m2) | 4,4 ± 2,1 |
| Mean Excess Weight Loss (%) | 48% ± 33 |
| Mean Weight loss (kg) | 12,2 ± 5,7 |
This level of efficacy is similar to other balloons6,7,8,9,10 present on the market.
This has been demonstrated by Palmisano in her study from 2016 where she did not find a significative difference between BIB and the Heliosphere balloon.
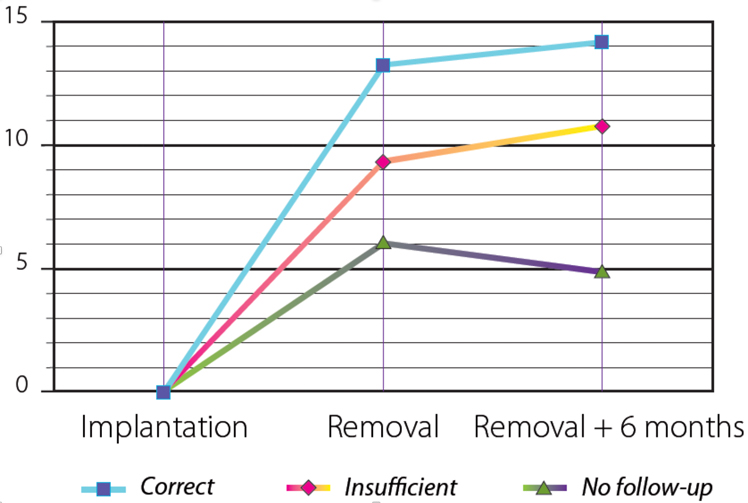
This has been previously shown by Costil11 in her comparative study which concluded that results were similar between both the Heliosphere ballon and BIB but also highlighted the decisive aspect of the quality of patient monitoring to achieve real and lasting weight loss.
The following figure 1 shows the difference in terms of weight loss according to the quality of the follow up:
| Balloon | Study | Weight loss(kg) |
|---|---|---|
| Silimed | Guedes, E.P., et al.(2016). Diabetol Metab Syndr 8: 81. | 11,7 ± 9,6 |
| Obalon | De Peppo, F., et al.(2017). Endosc Int Open 5(1): E59-E63 | 6.54 |
| MedSil | Almeghaiseeb, E. S., et al.(2017).World J Clin Cases 5(4): 140-147 | 12,48 ± 5,16 |
Orbera Reshape | Tate, C.M.etal.(2107). Adv Ther 34(8): 1859-1875 | 9,9 ± 6,6 (Etude FDA 2015) 7,2 ± 5,4 |
Fig. 1
Tolerance
The most important difference concerns tolerance and this is also in favour of the Heliosphere air inflated intragastric balloon. The usual adverse events are known for other intragastric balloon types :
| Balloon | Study | Deflation | Migration | Early Removal |
|---|---|---|---|---|
| Silimed | Guedes, E.P., et al.(2016). Diabetol Metab Syndr 8: 81. | NR | NR | 8% |
| Obalon | De Peppo, F., et al.(2017). Endosc Int Open 5(1): E59-E63 | 31% | 18% | NR |
| MedSil | Almeghaiseeb, E. S., et al.(2017).World J Clin Cases 5(4): 140-147 | NR | 2% (2 surgical remove) | 7% |
| Orbera | Force A.B.E.T. et al(2015). Gastrointest Endosc 82(3):425 | NR | 1.4% | 7.5% |
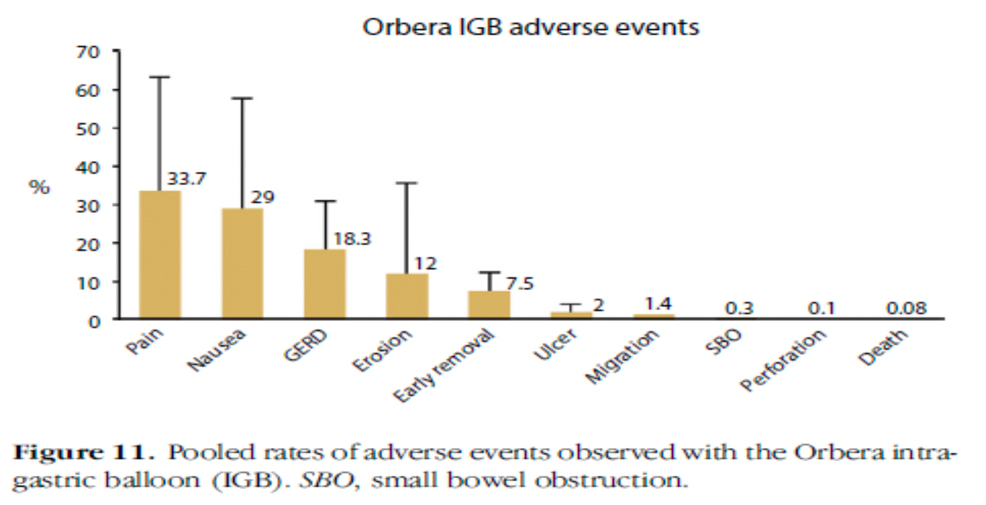
It should be noted that results with the Heliosphere balloon are clearly different with a low rate of adverse events and impressive findings regarding the safety of use.
| Study | Number of patients with Heliosphere | Inplantation duration | Deflation | Migration | Early Removal |
|---|---|---|---|---|---|
| Erdem, H., et al.(2016). Dicle Tip Dergisi 43(1) | 75 | 26 weeks | 4 in the last month | 0 | 3 removal for intolerance |
| Greco (Kaizer Hospital – Soa Paulo) | 10 | 25 weeks | 0 | 0 | 0 |
| Palmisano, S., etal.(2016). Obesity surgery 26(9): 2131-2137. | 58 | 24 weeks | NR | 0 | 0 |
| Espinet Coll, E., etal.(2017). Rev Esp Enferm Dig 109(5): 350-357 | 70 | 24 weeks | Few (no figures) | 0 | NR |
| Study | Erdem, H., et al.(2016). Dicle Tip Dergisi 43(1) |
|---|---|
| Number of patients with Heliosphere | 75 |
| Inplantation duration | 26 weeks |
| Deflation | 4 in the last month |
| Migration | 0 |
| Early Removal | 3 removal for intolerance |
| Study | Greco (Kaizer Hospital – Soa Paulo) |
|---|---|
| Number of patients with Heliosphere | 10 |
| Inplantation duration | 25 weeks |
| Deflation | 0 |
| Migration | 0 |
| Early Removal | 0 |
| Study | Palmisano, S., etal.(2016). Obesity surgery 26(9): 2131-2137. |
|---|---|
| Number of patients with Heliosphere | 58 |
| Inplantation duration | 24 weeks |
| Deflation | NR |
| Migration | 0 |
| Early Removal | 0 |
| Study | Espinet Coll, E., etal.(2017). Rev Esp Enferm Dig 109(5): 350-357 |
|---|---|
| Number of patients with Heliosphere | 70 |
| Inplantation duration | 24 weeks |
| Deflation | Few (no figures) |
| Migration | 0 |
| Early Removal | NR |
Safety
- Death (33 between 2006 and 2017) with liquid-filled balloons are underestimated12.
- There is a real problem with over-inflation and pancreatitis with liquid filled balloon.13
- Medsil still has problem of ulceration and migration14,15.
- Elipse still have seducing results in terms of weight loss (13kg)16 and begins to know about problems of overinflation17.
- Spatz 3 is still dealing with erosion/perforation leading up to 20% of early removal18,19,20 and pancreatitis 21.
- Other liquid filled balloons (quoted above) remain with around 8% early removal.
- 4 new deaths declared.
- 40% are mentioned as injuries.
- ¼ are due to over inflation (up to 1.2 liters).
At the same time, the Heliosphere balloon demonstrates safe use for the practitioner and the patient every time. The proof is clear that there is no need to make patients sick so that they lose weight.
Bibliography
1 A Erdem, H., et al. (2016). “Effects of Intragastric Balloon on Body Mass Index, Lipid Profile and Blood Glucose Regulation: A Prospective Study.” Dicle Tıp Dergisi 43(1).
2 Palmisano, S., et al. (2016). “Intragastric Balloon Device: Weight Loss and Satisfaction Degree.” Obesity surgery 26(9): 2131-2137.
3 Espinet Coll, E., et al. (2017). “Multicenter study on the safety of bariatric endoscopy.” Rev Esp Enferm Dig 109(5): 350-357.
4 Romney (2020), Single center prospective cohort on Heliopshère Newtech (on going)
5 Coenye (2017), Single center prospective cohort on Heliopshère Newtech (CREGG 2017)
6 Guedes, E. P., et al. (2016). “Impact of a 6-month treatment with intragastric balloon on body composition and psychopathological profile in obese individuals with metabolic syndrome.“ Diabetol Metab Syndr 8: 81.
7 De Peppo, F., et al. (2017).”The Obalon swallowable intragastric balloon in pediatric and adolescent morbid obesity.“Endosc Int Open 5(1): E59-E63
8 Almeghaiseeb, E. S., et al. (2017). “Efficacy of intragastric balloon on weight reduction: Saudi perspective.” World J Clin Cases 5(4): 140-147
9 Tate, C. M. and A. Geliebter (2017). “Intragastric Balloon Treatment for Obesity: Review of Recent Studies.” Adv Ther 34(8): 1859-1875.
10 Force, A. B. E. T., et al. (2015). “ASGE Bariatric Endoscopy Task Force systematic review and meta-analysis assessing the ASGE PIVI thresholds for adopting endoscopic bariatric therapies.” Gastrointest Endosc 82(3): 425-438 e424
11 Costil, V. (2009). Gastric balloon efficiency on weight loss with a multidisciplinary medical follow-up (Poster at ASMBS)
12 Tate, C. M., et al. (2018). Intragastric balloon treatment for obesity FDA safety updates. Advances in therapy, 35(1), 1-4
13 FDA – Consumer Update : Medical Devices that Treat Obesity: What to Know (June 2018), www.fda.gov
14 Kool, N., & Müggler, S. A. (2018). Gastric outlet obstruction: a rare complication in patients with intragastric balloon treatment for obesity. BMJ case reports, 2018.
15 Mojkowska, A., Gazdzinski, S., Fraczek, M., & Wyleżoł, M. (2017). Gastric Ulcer Hemorrhage-a Potential Life-Threatening Complication of Intragastric Balloon Treatment of Obesity. Obesity facts, 10(2), 153-159.
16 Ernesti I, Ienca R, Basciani S, Mariani S, Genco A (2018) Effect of A New Swallowable Intragastric Balloon (Elipse™) on Weight Loss and Metabolic Syndrome. J Obes Nutr Disord: JOND-120
17 Allurion Tech, Field Safety Notice in France Nov. 20th, 2018
18 Espinet-Coll, E., Nebreda-Duran, J., López-Nava-Breviere, G., Ducons-Garcia, J., Rodriguez-Tellez, M., Crespo-García, J., & Marra-Lopez-Valenciano, C. (2017). Multicenter study on the safety of bariatric endoscopy. Rev Esp Enferm Dig, 109(5), 350-357.
19 Rahman, A. A., & Loi, K. (2018). Gastric Perforation as a complication of intragastric balloon. Surgery for Obesity and Related Diseases, 14(5), 719-722.
20 Dayan, D., Sagie, B., & Fishman, S. (2016). Late intragastric balloon induced gastric perforation. Obesity surgery, 26(5), 1138-1140
21 Gore, N., Ravindran, P., Chan, D. L., Das, K., & Cosman, P. H. (2018). Pancreatitis from intra-gastric balloon insertion: Case report and literature review. International journal of surgery case reports, 45, 79-82.
